Written by Norman T. Neher, P.E.
Analytical Engineering Services
Introduction
Developing new medical devices is both costly and time-consuming. Prototyping, bench testing, and redesign cycles can stretch development timelines and budgets. Finite Element Analysis (FEA) offers a way to break this cycle by enabling engineers to virtually test and refine designs early in the process.
In catheter development, FEA provides benefits to evaluate how a device behaves under realistic conditions before a prototype is ever built. This reduces physical test cycles, accelerates timelines, and lowers costs—while offering insights that traditional testing cannot capture.
Why FEA Matters in Catheter Design
Catheters must perform reliably in demanding environments, often navigating tortuous anatomy without kinking or buckling. Conventional trial-and-error prototyping requires months of design, fabrication, and bench testing. FEA streamlines this process by simulating real-world performance in a virtual environment.
By analyzing stress, strain, and deformation, engineers can quickly evaluate design variations, identify weak points, and optimize structures—long before committing resources to physical builds.
Key Design Variables
In a catheter design study, the most critical parameters often include:
- Sheath materials and wall thickness
- Cross-sectional geometry (diameter, thickness, braid spacing)
- Structural enhancements (reinforcement wires, braiding, or other stiffeners)
One powerful enhancement strategy is embedding wire reinforcements into the sheath. FEA allows designers to study how variables like wire material, strand number, cross-section shape, and pitch angle (from nearly 0° to 90°) influence catheter performance.
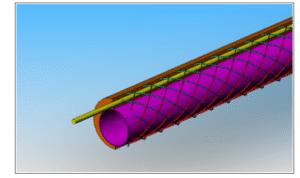
Cutaway view of embedded wire reinforcements with inner sheath and guide wire
The Analysis Process
The typical workflow for FEA in catheter design includes:
- 3D CAD Modeling – Build the geometry of the catheter, including sheaths, braid, and reinforcement features.
- Material Properties Input – Define mechanical behavior, including nonlinear elasticity of flexible polymers.
- Assembly Setup – Virtually bond, sliding , or otherwise constrain parts to mimic real interactions.
- Meshing – Divide the model into thousands of discrete elements, allowing stress and strain distribution across the structure.
- Boundary Conditions – Apply realistic constraints and forces (e.g., bending radius, torsional loading).
- Execution & Results – Run simulations to obtain localized stresses, strains, and deformation profiles.
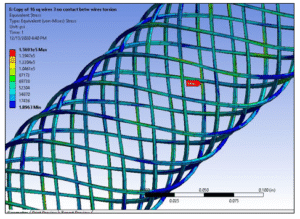
Unlike physical tests, FEA can capture data inside the structure (e.g., stresses in an inner braid wire) that is otherwise impossible to measure.
View of stresses within reinforcement wires
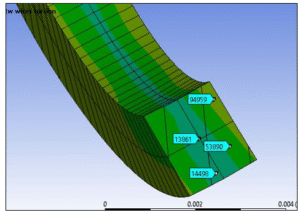
Reinforcement wire cross section stresses
Reducing Test Burden with DOE
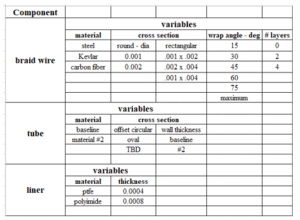
Testing every possible variable combination could require hundreds of FEA runs. Instead, engineers apply Design of Experiments (DOE) methodology to reduce the matrix to a manageable number of cases while still covering the design space.
This allows focused exploration of the most promising designs—balancing accuracy with efficiency.
Sample Test Matrix. Can be greatly simplified using DOE
Minimum Bend Radius and Failure Modes
A key performance metric for catheters is minimum bend radius—the tightest curvature a catheter can navigate without kinking, buckling, or exceeding material elastic limits. FEA provides a fast way to predict these thresholds across different design variations, enabling teams to select the best candidates for physical testing.
Summary
Finite Element Analysis is a powerful tool for medical device development, particularly in catheter design. By virtually testing multiple design variations before building prototypes, teams can:
- Shorten development cycles
- Lower costs by reducing physical test iterations
- Explore a wider range of design concepts
- Gain insight into internal stresses not measurable in bench tests
For companies developing complex catheter systems, FEA provides a competitive edge—delivering better designs, faster and at lower cost.