Analysis
Medical Devices
How Finite Element Analysis is Transforming Medical Device Development
Developing medical devices is expensive, time-consuming, and highly regulated. Traditionally, teams rely on a cycle of design, build, and test to get products ready for certification and manufacturing. But there’s a smarter way to work: Finite Element Analysis (FEA).
FEA lets engineers simulate how a device will behave under real-world conditions—before a single prototype is built. The results? Fewer physical prototypes, faster iterations, and lower development costs. And the benefits extend well beyond design: FEA can improve manufacturing processes, optimize assembly, support quality control, troubleshoot product issues, simulate transportation or drop tests, and even guide future product extensions. In short, it’s a tool that adds value throughout a device’s entire lifecycle.
A blog article here goes into much more detail.
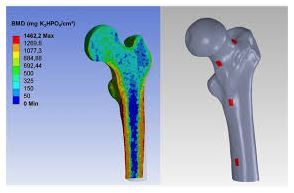
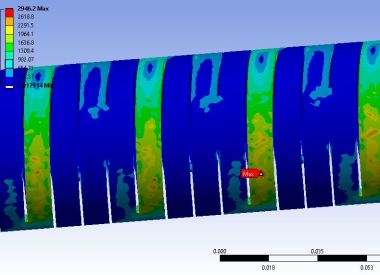
Laser Cut SS Catheter Stress
Take a recent catheter development project. The manufacturer followed its standard design-build-test cycle, which took four months to source and produce a prototype and gather basic performance data: flex and torsion characteristics of a hypodermic tube modified with micro-cuts. Several cut patterns were tested to see which worked best.
Meanwhile, engineers ran a parallel FEA simulation using the same CAD models. The simulation took just two days—and the results closely matched the months of physical testing.
This example shows the potential of FEA: what traditionally took months could have been predicted accurately in a matter of days, saving time, resources, and costs, and enabling engineers to explore more design options faster.
Why Medical Device Teams Should Embrace FEA
-
Accelerated Development: Reduce reliance on physical prototypes and speed up iterations.
-
Cost Savings: Cut down expensive testing cycles and materials waste.
-
Better Insights: Understand stress, flex, and performance characteristics in detail.
-
Lifecycle Support: Apply simulations to manufacturing, transport, and even product extensions.
FEA is no longer just a nice-to-have tool—it’s a game-changer for medical device development. Teams that leverage simulation early can innovate faster, reduce costs, and bring safer, more reliable products to market.